Alvotechs Strategic Moves Analyzing the Investment Potential in Biosimilars Market

Hey fellow investors! Kane Buffett here with another deep dive into the biopharmaceutical sector. Today, we’re examining Alvotech’s recent regulatory developments that could significantly impact their market position and stock performance. As we navigate this complex landscape, I’ll break down what these announcements mean for your investment portfolio and why biosimilars represent one of the most promising areas in healthcare investing right now.
☁️ Want to stay ahead of the market with data-driven investment strategies? Here’s what you need to know about BIPC Stock Why This Infrastructure Giant Could Deliver Massive Earnings Surprise for comprehensive market insights and expert analysis.
MHRA Approval: Gobivaz Biosimilar Marks European Expansion
The recent marketing authorization from the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) for Gobivaz, a biosimilar to Simponi (golimumab), represents a significant milestone for Alvotech and their partner Advanz Pharma. This approval covers the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis - conditions affecting millions worldwide. The European biosimilars market is projected to reach $16 billion by 2027, growing at a CAGR of 24.3%. This approval positions Alvotech to capture substantial market share as biosimilar adoption accelerates across European healthcare systems seeking cost containment. The timing is particularly strategic given the upcoming patent expirations of several blockbuster biologics. From an investment perspective, this regulatory success demonstrates Alvotech’s capability to navigate complex approval processes and establishes their credibility in the global biosimilars landscape. The partnership with Advanz Pharma, which has established commercial infrastructure across Europe, provides immediate market access and reduces the capital expenditure typically required for market entry. This collaborative approach could become a blueprint for future market expansions.
Searching for a fun and engaging puzzle game? Sudoku Journey with Grandpa Crypto’s story offers a unique twist on classic Sudoku.
US Regulatory Landscape: AVT05 BLA Status Update
Alvotech’s recent update on their Biologics License Application (BLA) for AVT05 in the United States reveals both challenges and opportunities. While the company continues to work through the regulatory process with the FDA, investors should understand that such regulatory journeys are typical in the biopharmaceutical industry. The US represents the largest pharmaceutical market globally, with biosimilars expected to generate $30 billion in savings by 2026. AVT05 targets a significant therapeutic area, and successful approval could open substantial revenue streams. However, the regulatory pathway requires careful navigation of complex requirements including manufacturing standards, clinical data submission, and compliance with the Biologics Price Competition and Innovation Act. The current status update indicates ongoing dialogue with regulators, which is generally positive as it demonstrates active progress toward resolution. For long-term investors, these regulatory processes, while sometimes lengthy, represent necessary steps toward market entry. The experience gained through these interactions strengthens the company’s regulatory capabilities and positions them better for future submissions. The potential market size for AVT05’s target indication makes this regulatory journey worth monitoring closely.
Take your Powerball strategy to the next level with real-time stats and AI predictions from Powerball Predictor.
Investment Analysis: Biosimilars Market Dynamics and Alvotech’s Position
The global biosimilars market presents a compelling investment thesis driven by multiple factors: healthcare cost pressures, patent expirations of originator biologics, and increasing regulatory clarity worldwide. Alvotech’s focused strategy on developing high-quality biosimilars positions them to capitalize on these trends. Their pipeline includes multiple candidates targeting blockbuster biologics with combined annual sales exceeding $50 billion. The company’s integrated approach - controlling development and manufacturing - provides cost advantages and quality control that could translate to competitive pricing and higher margins. From a market perspective, biosimilar adoption is accelerating globally as payers and providers seek sustainable healthcare solutions. The Inflation Reduction Act in the US and similar initiatives in Europe are creating favorable conditions for biosimilar uptake. However, investors must consider the competitive landscape, including established players like Sandoz and emerging competitors. Alvotech’s partnership strategy with regional experts like Advanz Pharma demonstrates smart capital allocation and risk management. The current valuation multiples in the biosimilars sector suggest potential upside, particularly for companies with near-term regulatory catalysts and established manufacturing capabilities.

Looking for a game to boost concentration and brain activity? Sudoku Journey: Grandpa Crypto is here to help you stay sharp.
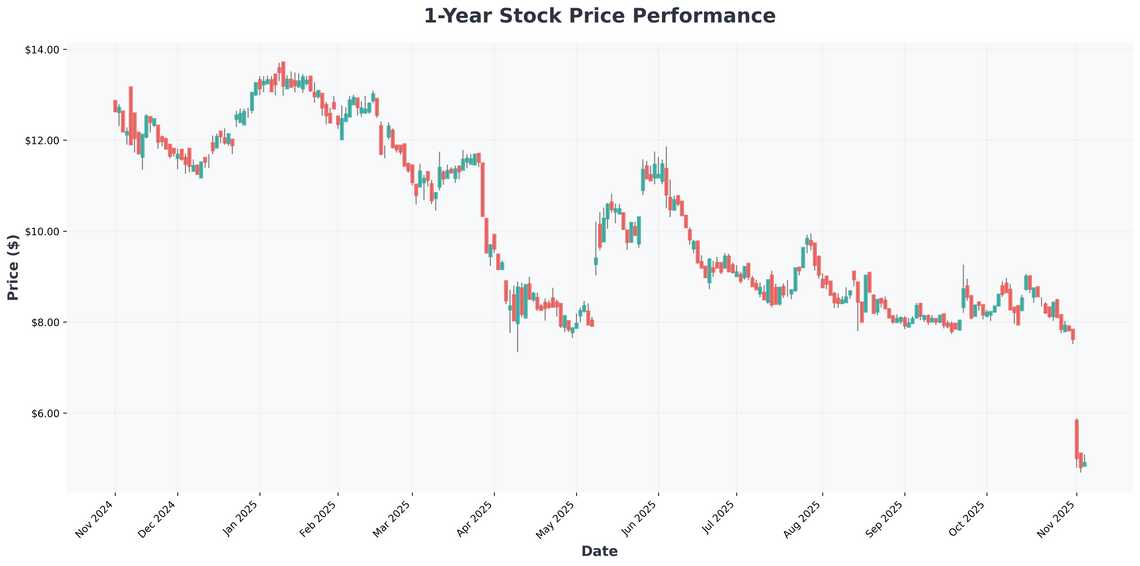
In conclusion, Alvotech’s recent regulatory developments represent important milestones in their growth trajectory. While regulatory processes require patience, the underlying market dynamics for biosimilars remain strongly favorable. The MHRA approval for Gobivaz establishes their European presence, while ongoing work on US approvals maintains their global ambitions. As always in biotech investing, diversification and long-term perspective are key. The biosimilars revolution is just beginning, and companies like Alvotech that can successfully navigate regulatory pathways and build commercial partnerships could deliver substantial returns. Keep watching this space, and remember - in volatile sectors like biotech, timing your entries and maintaining conviction through regulatory uncertainty often separates successful investors from the rest. Happy investing!
If you need to create custom QR codes with logo integration and color options, this free QR code generator offers everything in one place.